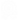Dr. Deepak Chitkara is an associate professor at the Department of Pharmacy, Pilani Campus, BITS Pilani, and his lab is actively working on CRISPR/Cas gene editing tools for therapeutic and diagnostic applications. They are developing nanotechnology-based products to facilitate the efficient delivery of CRISPR for the treatment of debilitating diseases. The lab also focuses on developing CRISPR-based biosensors for early-stage diagnosis.
CRISPR/Cas9 is a molecular scissor that could provide precise and site-specific gene editing via double-strand break (DSB) mediated non-homologous end joining (NHEJ) or homology-directed repair (HDR) repair pathways (Figure 1a). The technology has shown immense potential as a therapeutic tool for treating uncurable genetic or non-genetic diseases. However, the use of the CRISPR/Cas tool is limited due to its high molecular weight, negative charge, and instability in the presence of nucleases or proteases, etc. (Figure 1b). Since 2017, Dr. Chitkara’s lab has been actively developing non-viral lipidic and polymeric nano-carriers to deliver CRISPR components, specifically the ribonucleoprotein (RNPs) complexes. We have developed lipo-polymeric carriers based on a polycarbonate backbone that stabilizes and effectively delivers these ribonucleoproteins to the cells and in animal models.
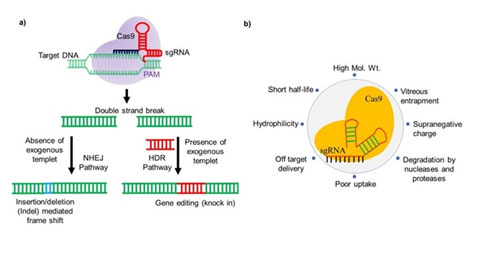
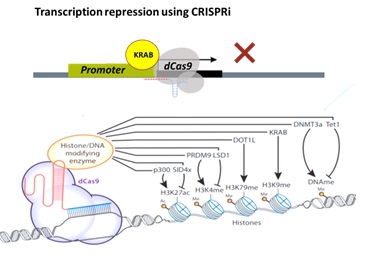
Figure 2. CRISPR dCas9 mediated epigenome editing to control gene expression
Recently, the Cas effector of class V of the CRISPR/Cas system, namely Cas12 and Cas13, are reported for their trans-cleavage activity that could be utilized for diagnostic applications. We are developing a CRISPR biosensor for early-stage diagnosis of diabetic nephropathy wherein the changes in the cell-free DNA (cfDNA) in the blood and urine could reflect the pathological condition and associated complications. CRISPR biosensors have been reported for their ability to detect nucleic acid at the femtomole level with high accuracy and precision. Therefore, they could be a potential method to detect nucleic acid biomarkers. Interrogation of the cell-free DNA isolated from the blood and urine samples could be used to diagnose a disease progression. Briefly, the Cas12 RNPs are designed to identify a specific gene in the cell-free DNA that could directly correlate with the disease progression (Figure 3).
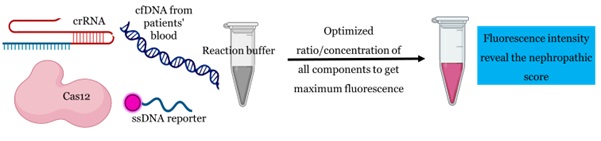
Figure 3. CRISPR/Cas based biosensor for diagnostic applications
CRISPR technology has shown immense potential for therapeutic and diagnostic applications. Our lab’s focus is to innovate and translate advanced healthcare products on CRISPR technology for societal benefits.
You Might Also Like
- BITS to Baker Hughes: Shad Hussain’s Journey Through Turbulent Times
- Be Cool: Navigating Life, Literature, and Mental Health with Shashi Warrier
- Coaching Across Cultures: Brajesh Bajpai’s Journey of Learning, Leading, and Adapting
- From Engineer to AI Leader: An Interview with Akhil Singhal on Startups, Strategy, and the Future of Gen A
- From Circuits to Capital Markets: Deepak Joshi’s Global Finance Journey